News
News
Featured Products
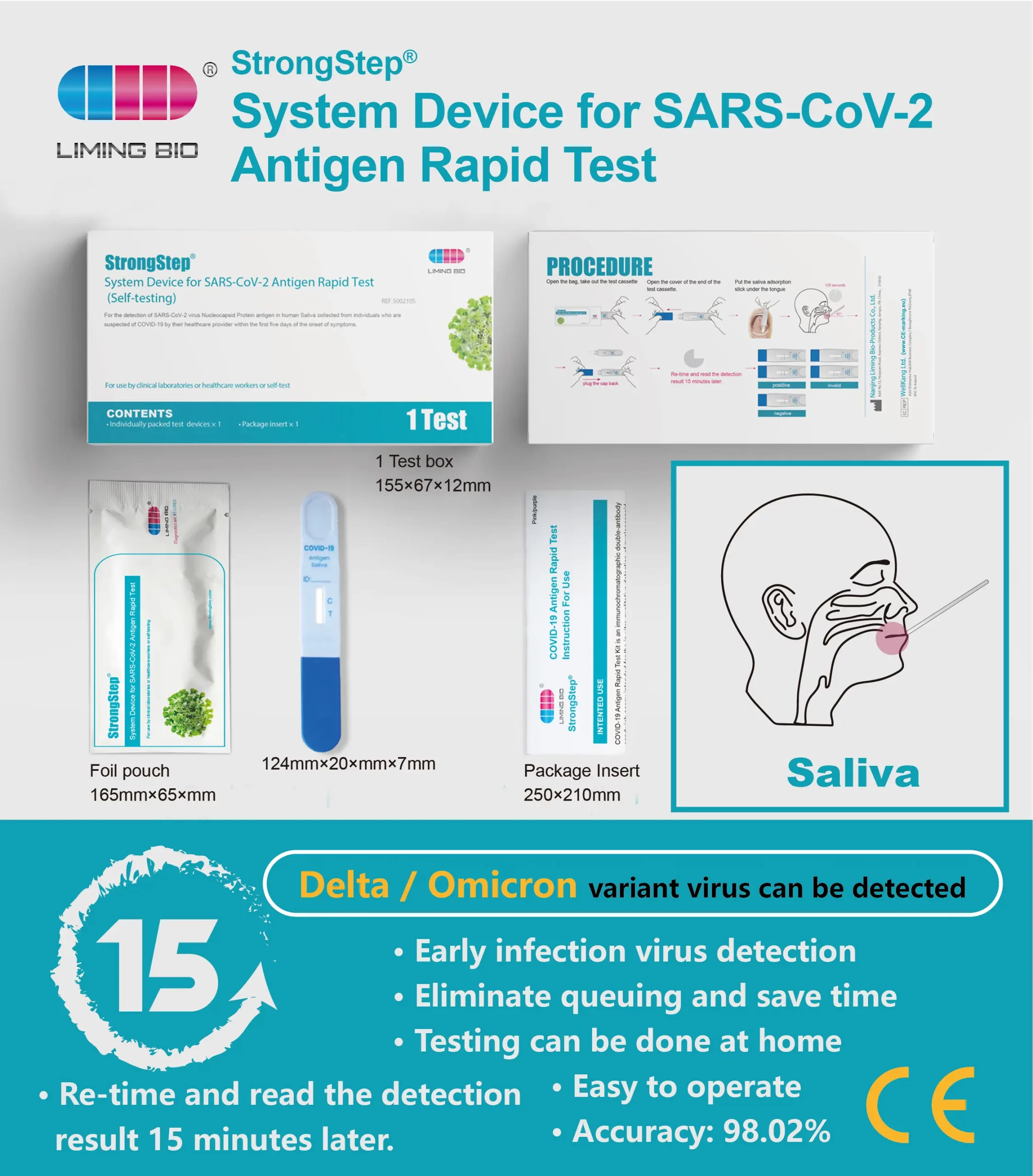
StrongStep System Device for SARS-CoV-2Antigen Rapid Test employs immunochromatography technobgy to detect the SARS-CoV-2 nucleocapsid antigen in human saliva. This test is single use only and intended for self-testing. It is recommended to use this test within 7 days of symptom onset. lt is supported by the dinical performance assessment.
| REF | 500160 | Specification | 20 Tests/Box |
| Detection principle | Immunochromatographic assay | Specimens | Cervicovaginal secretions |
| Intended Use | StrongStep® Fetal Fibronectin Rapid Test is a visually interpreted immunochromatographic test intended to be used for the qualitative detection of fetal fibronectin in cervicovaginal secretions. | ||
| REF | 500020 | Specification | 20 Tests/Box |
| Detection principle | Immunochromatographic assay | Specimens | Cervical/urethra swab |
| Intended Use | It is suitable for qualitative detection of gonorrhoea/chlamydia trachomatis antigens in cervical secretions of women and urethral samples of men in vitro in various medical institutions for auxiliary diagnosis of the above pathogen infection. | ||
| REF | 501080 | Specification | 20 Tests/Box |
| Detection principle | Immunochromatographic assay | Specimens | Feces |
| Intended Use | The StrongStep® Salmonella Antigen Rapid Test is a rapid visual immunoassay for the qualitative, presumptive detection of Salmonella typhimurium, Salmonella enteritidis, Salmonella choleraesuis in human fecal specimens. This kit is intended for use as an aid in the diagnosis of Salmonella infection. | ||
| REF | 500080 | Specification | 50 Tests/Box |
| Detection principle | PH value | Specimens | Vaginal discharge |
| Intended Use | The StrongStep® Bacterial vaginosis(BV) Rapid Test Device is intend to measure the vaginal pH for aid in the diagnosis of Bacterial vaginosis. | ||
For the qualitative detection of Trichomonas and/or Candida and/or Gardnerella Vaginalis antigens from vaginal swabs or from the saline solution prepared when making wet mount from vaginal swabs. This kit is intended to be used as an aid in the diagnosis of Candida albicans and/or Trichomonas Vaginalis andlorGardnerella Vaginalis infection.