Product
Product Categories
Featured products
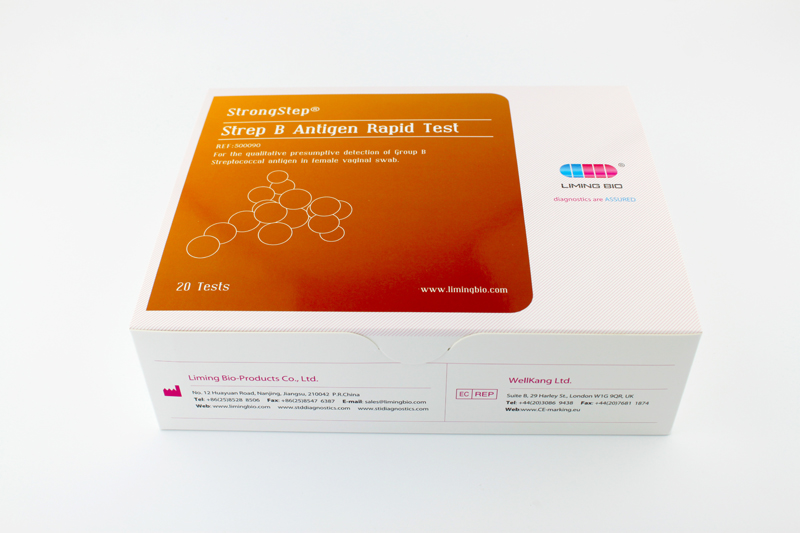
| REF | 500090 | Specification | 20 Tests/Box |
| Detection principle | Immunochromatographic assay | Specimens | Female vaginal swab |
| Intended Use | StrongStep® Strep B antigen Rapid Test is a rapid visual immunoassay for the qualitative presumptive detection of Group B Streptococcal antigen in female vaginal swab. | ||
This is a rapid immuno-chromatographic assay for the simultaneous detection of IgM and IgG antibodies to SARS-CoV-2 virus in human whole blood, serum or plasma.
| REF | 500040 | Specification | 20 Tests/Box |
| Detection principle | Immunochromatographic assay | Specimens | Vaginal discharge |
| Intended Use | StrongStep® Trichomonas vaginalis antigen rapid test is a rapid lateral-flow immuno assay for the qualitative detection of Trichomonas vaginalis antigens in vaginal swab. | ||
| REF | 500170 | Specification | 20 Tests/Box |
| Detection principle | Immunochromatographic assay | Specimens | Vaginal discharge |
| Intended Use | StrongStep® PROM rapid test is a visually interpreted, qualitative immunochromatographic test for detection of IGFBP-1 from amniotic fluid in vaginal secretions during pregnancy. | ||
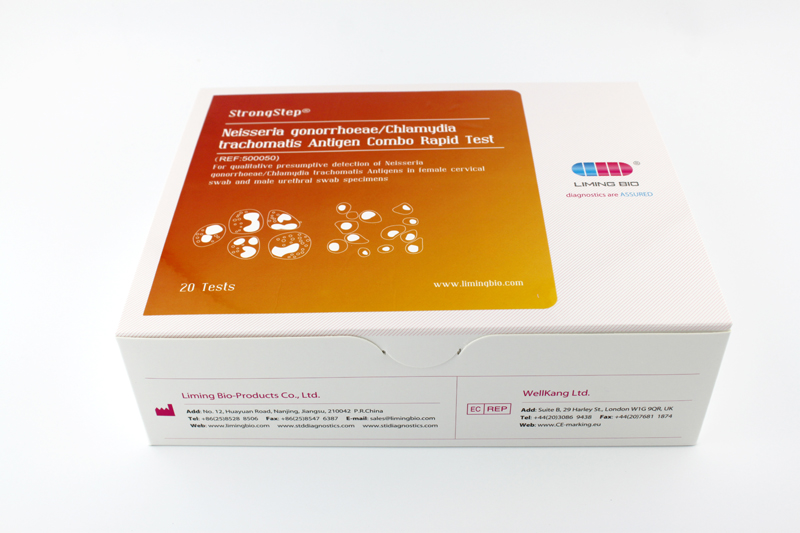
| REF | 500050 | Specification | 20 Tests/Box |
| Detection principle | Immunochromatographic assay | Specimens |
Cervical/urethra swab |
| Intended Use | This is a rapid lateral-flow immunoassay for the qualitative presumptive detection of Neisseria gonorrhoeae/Chlamydia trachomatis antigens in male urethral and female cervical swab | ||
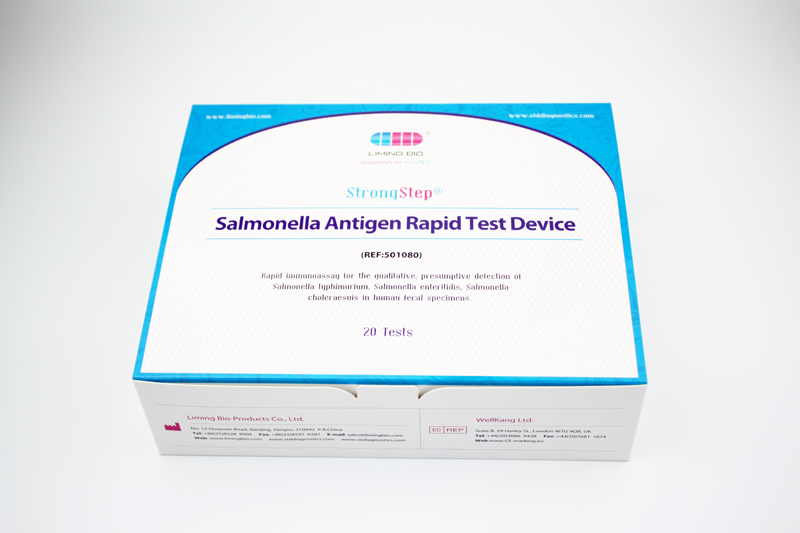
| REF | 501080 | Specification | 20 Tests/Box |
| Detection principle | Immunochromatographic assay | Specimens | Feces |
| Intended Use | The StrongStep® Salmonella Antigen Rapid Test is a rapid visual immunoassay for the qualitative, presumptive detection of Salmonella typhimurium, Salmonella enteritidis, Salmonella choleraesuis in human fecal specimens. This kit is intended for use as an aid in the diagnosis of Salmonella infection. | ||
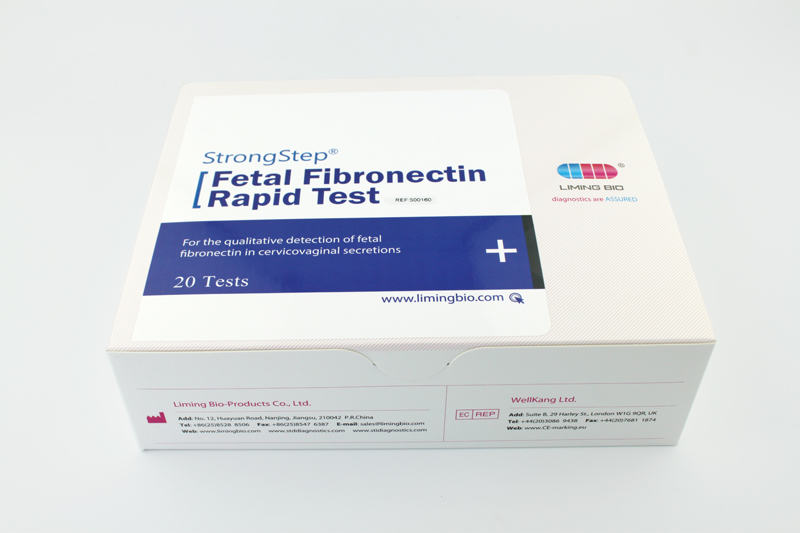
| REF | 500160 | Specification | 20 Tests/Box |
| Detection principle | Immunochromatographic assay | Specimens | Cervicovaginal secretions |
| Intended Use | StrongStep® Fetal Fibronectin Rapid Test is a visually interpreted immunochromatographic test intended to be used for the qualitative detection of fetal fibronectin in cervicovaginal secretions. | ||
| REF | 500170 | Specification | 20 Tests/Box |
| Detection principle | Immunochromatographic assay | Specimens | Vaginal discharge |
| Intended Use | StrongStep® PROM rapid test is a visually interpreted, qualitative immunochromatographic test for detection of IGFBP-1 from amniotic fluid in vaginal secretions during pregnancy. | ||
| REF | 501100 | Specification | 20 Tests/Box |
| Detection principle | Immunochromatographic assay | Specimens | Feces |
| Intended Use | StrongStep® Giardia lamblia Antigen Rapid Test Device (Feces) is a rapid visual immunoassay for the qualitative, presumptive detection of Giardia lamblia in human fecal specimens. This kit is intended for use as an aid in the diagnosis of Giardia lamblia infection. | ||
| REF | 501080 | Specification | 20 Tests/Box |
| Detection principle | Immunochromatographic assay | Specimens | Feces |
| Intended Use | The StrongStep® Salmonella Antigen Rapid Test is a rapid visual immunoassay for the qualitative, presumptive detection of Salmonella typhimurium, Salmonella enteritidis, Salmonella choleraesuis in human fecal specimens. This kit is intended for use as an aid in the diagnosis of Salmonella infection. | ||
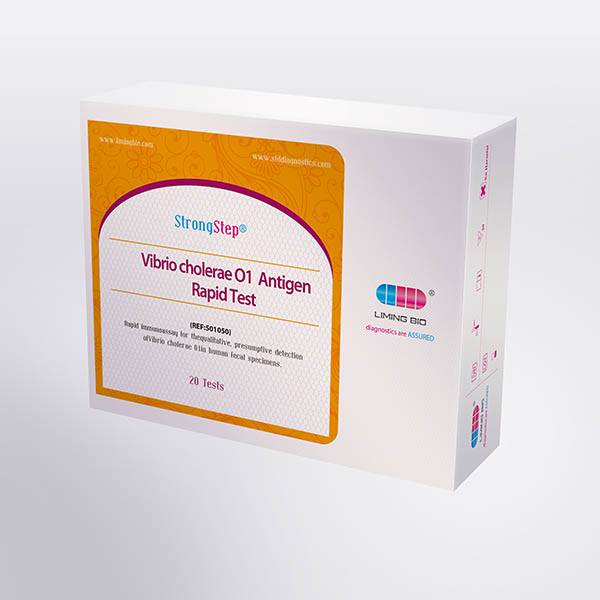
| REF | 501050 | Specification | 20 Tests/Box |
| Detection principle | Immunochromatographic assay | Specimens | Feces |
| Intended Use | The StrongStep® Vibrio cholerae O1 Antigen Rapid Test Device (Feces) is a rapid visual immunoassay for the qualitative, presumptive detection of Vibrio cholerae O1 in human fecal specimens. This kit is intended for use as an aid in the diagnosis of Vibrio cholerae O1 infection. | ||
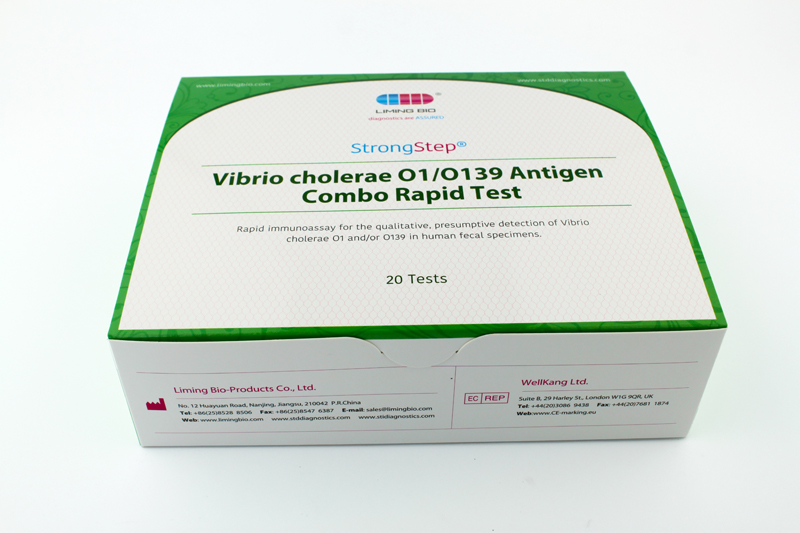
| REF | 501070 | Specification | 20 Tests/Box |
| Detection principle | Immunochromatographic assay | Specimens | Feces |
| Intended Use | The StrongStep® Vibrio cholerae O1/O139 Antigen Combo Rapid Test is a rapid visual immunoassay for the qualitative, presumptive detection of Vibrio cholerae O1 and/or O139 in human fecal specimens. This kit is intended for use as an aid in the diagnosis of Vibrio cholerae O1 and/or O139 infection. | ||